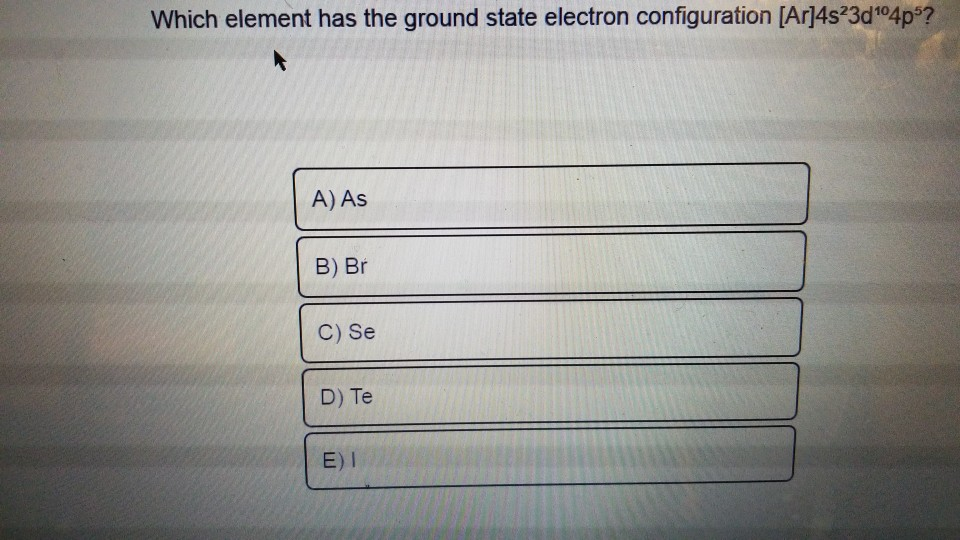
Question
Asked By VelvetWhisper68 at
Answered By Expert
Derrick
Expert · 1.5k answers · 1k people helped
Given electronic configuration is
[Ar]4s23d104p5
[Ar] is corresponds to Argon and it has 18 electrons. In 4S orbital, there are 2 electrons, 3d orbital there are 10 electrons and 4p orbital, there are 5 electrons.
Total number of electrons = 18+2+10+5 = 35.
We know that atomic number (Z) is the number of electrons or number of protons present in an atom.
Therefore atomic number of given element is 35.
It is corresponds to Bromine(Br)
Option-B is the correct answer.
Option-A: Arsenic (As) atomic number-33. Number of electrons are 33
Option-C: Selenium- Atomic number 34. Number of electrons are 34
Option D: Tellurium (Te): Atomic number 52. Number of electrons are 52
Option E: Iodine: Atomic number is 53. Number of electrons are 53.
Option B is the correct answer.
🧑🏫 More Questions
👉 Interested in exploring further?
Chrome Extension
1. Search answers from our 90+ million questions database.
2. Get instantly AI Solutions powered by most advanced models like GPT-4, Bard, Math GPT, etc.
3. Enjoy one-stop access to millions of textbook solutions.
4. Chat with 50+ AI study mates to get personalized course studies.
5. Ask your questions simply with texts or screenshots everywhere.