Question
Asked By ShadowDancer67 at
Answered By Expert
Vincent
Expert · 1.5k answers · 1k people helped
Step 1/2
Lewis Structure:
A Lewis structure is a representation of the valence electrons in an atom and how they are arranged in a molecule.
Explanation:
It uses the dots to show the valence electrons around atoms and lines to show the covalent bonds. The goal is to show the distribution of electrons in a way that satisfies the octet rule for most elements.
Step 2/2
Group Number and Valence Electrons:
In the periodic table, the column number refers to the number of valence electrons an element possess. Elements present in the same column will carry same chemical properties.
Explanation:
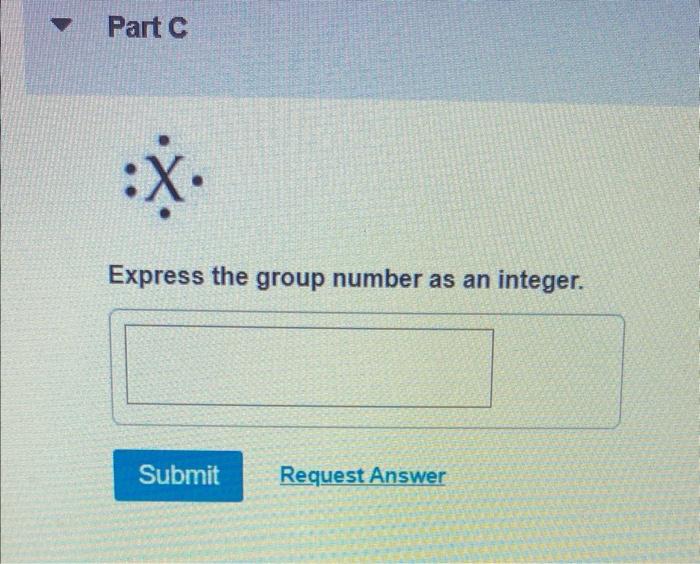
If an element has a total of five valence electrons, it is likely in Group 15 (also known as Group V-A). This group includes nitrogen `(N)` , phosphorus `(P)` , arsenic `(As)` , antimony `(Sb)` , and bismuth (Bi), among others. Each element in this group has five valence electrons in its outermost shell.As we can see the Lewis dot structure in the given question that it has five valence electrons means the outermost shell carries five electrons which totally refers to the group number means it will present in the 15th group.
Final Answer
Therefore the group number of this element is 15
👉 Interested in exploring further?
Chrome Extension
1. Search answers from our 90+ million questions database.
2. Get instantly AI Solutions powered by most advanced models like GPT-4, Bard, Math GPT, etc.
3. Enjoy one-stop access to millions of textbook solutions.
4. Chat with 50+ AI study mates to get personalized course studies.
5. Ask your questions simply with texts or screenshots everywhere.