Question
Asked By ShadowStarlight84 at
Answered By Expert
Brad
Expert · 3.2k answers · 3k people helped
Step 1/2
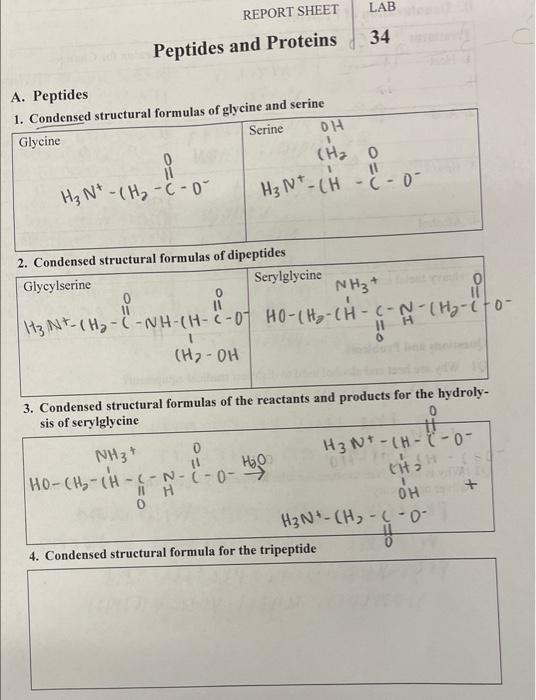
The objective of the question is to make condensed structural formula of given peptides and protien.
The expression "dense primary equation" alludes to a portrayal of a compound construction that passes on more data than a sub-atomic recipe however is less complex than a full underlying equation. Certain structural elements are written more concisely in a condensed structural formula, making it a shorthand notation for describing a molecule's atom arrangement.
Explanation:
Peptides are short chains of amino acids, which are the building blocks of proteins. Amino acids are organic compounds that contain an amino group (-NH2) and a carboxyl group (-COOH). When amino acids link together through peptide bonds, they form peptides.
Step 2/2
The condensed structure formulas of given peptides are as follows:
Serine:
Molecular Formula: `C3H7NO3`Condensed Structural Formula: ` CH₂OHCH(NH2)COOH``(2)~` Condensed structure formulas of dipeptides:Glycyl serine:
Structure: ` H2N-Gly-Ser-COOH`Serylglycine:
Structure: ` H2N-Ser-Gly-COOH ``(3)~` Condensed structure formulas of hydrolysis of Seryl-glycine:Serine: `H2N-Ser-COOH`Glycine: `H2N-Gly-COOH` `(4)~` Condensed structural formula for tripeptide.`H2N−CH2−CO-NH−CH-CH3−CO-NH-CHCH2OH-COOH``(5)~` Abbreviation for the tripeptide is given below as:Tripeptide made out of the amino acids Alanine `(Ala)` , Valine `(Val)` , and Leucine `(Leu)` :`One-` letter code: ` AVL``Three` -letter code: `Ala-Val-Leu`Explanation:
The hydrolysis of Serylglycine includes breaking the peptide connection between serine ( `Ser` ) and glycine ( `Gly` ), bringing about the arrangement of the singular amino acids.Final Answer
The condensed structural formula of given compounds is shown in step `2` .🧑🏫 More Questions
👉 Interested in exploring further?
Chrome Extension
1. Search answers from our 90+ million questions database.
2. Get instantly AI Solutions powered by most advanced models like GPT-4, Bard, Math GPT, etc.
3. Enjoy one-stop access to millions of textbook solutions.
4. Chat with 50+ AI study mates to get personalized course studies.
5. Ask your questions simply with texts or screenshots everywhere.