Question
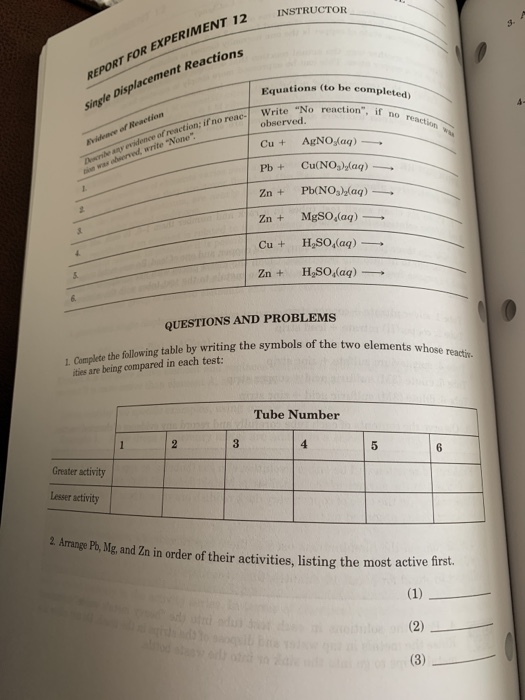
REPORT FOR EXPERIMENT 12 single Displacement Reactions INSTRUCTOR 12 s- Equations (to be completed) reaction", if no of reaction; if no reac- Write "No cti was obeerved, write "None Cu + HSO(aq) QUESTIONS AND PROBLEMS 1 Complete the follwing table by writing the symbols of the two elements ities are being compared in each test: 1. Complete the o elements whose Tube Number 5 Greater activity Lesser activity 2 P, M and Zn in order of their activities, listing the most activ first
NAME REPORT FOR EXPERIMENT 12 (continued) . Arrange Cu, Ag, and Zn in order of their activities, listing the most active firet 4. Arrange Mg. H, and Ag in order of their activities, listing the most active first. 5. Arrange all ive of the metals (excluding hyd rogen) in an activity series, listing the most active first. 6. On the basis of the reactions observed in the six test tubes, explain why the position of hydrogen cannot be fixed exactly with respect to all of the other elements listed in the activity series in Question 5. 7. What additional test(s) would be needed to establish the exact position of hyd the activity series of the elements listed in Question 5? 8. On the basis of the evidence developed in this experiment: (a) Would silver react with dilute sulfuric acid? Why or why not? b) Would magnesium react with dilute sulfuric acid? Why or why not?
Asked By EmeraldGemini84 at
Answered By Expert
Quinn
Expert · 5.4k answers · 5k people helped
🧑🏫 More Questions
👉 Interested in exploring further?
Chrome Extension
1. Search answers from our 90+ million questions database.
2. Get instantly AI Solutions powered by most advanced models like GPT-4, Bard, Math GPT, etc.
3. Enjoy one-stop access to millions of textbook solutions.
4. Chat with 50+ AI study mates to get personalized course studies.
5. Ask your questions simply with texts or screenshots everywhere.


