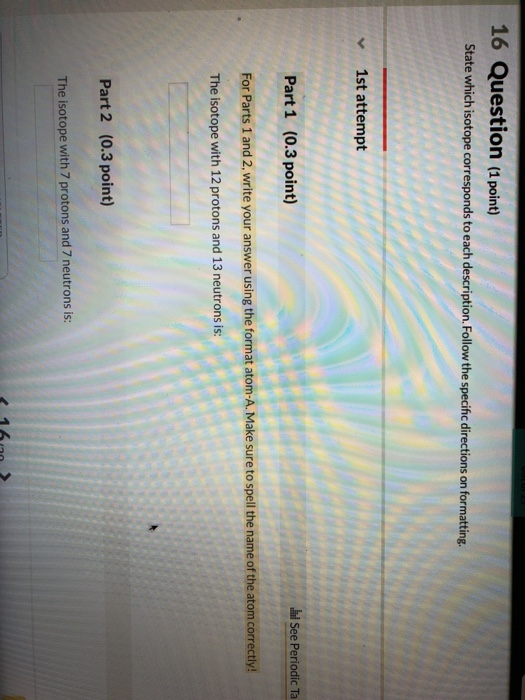
Question
Asked By GoldenLily32 at
Answered By Expert
Ryan
Expert · 3.2k answers · 3k people helped
Atomic number = number of protons
mass number = number of protons + number of neutrons
using atomic number we can identify the element from periodic table
part 1
number of protons = 12
number of neutrons=13
atomic number = 12
mass number = 12+13 =25
element with atomic number 12 = Magnesium
Magnesium- 12

********************************************
number of protons = 7
number of neutrons= 7
atomic number = 7
element with atomic number 7= Nitrogen
Nitrogen -7
mass number = 14

*******************************************
number of protons = 17
number of neutrons= 20
atomic number = 17
mass number = 37
element with atomic number 17 = Chlorine

*************************************
number of protons = 19
number of neutrons= 20
atomic number = 19
element with atomic number 19 = K
mass number Z= 39

***************
Thank you
🧑🏫 More Questions
👉 Interested in exploring further?
Chrome Extension
1. Search answers from our 90+ million questions database.
2. Get instantly AI Solutions powered by most advanced models like GPT-4, Bard, Math GPT, etc.
3. Enjoy one-stop access to millions of textbook solutions.
4. Chat with 50+ AI study mates to get personalized course studies.
5. Ask your questions simply with texts or screenshots everywhere.